Introduction. The combination of lenalidomide and rituximab (or R2) has been demonstrated to be an effective frontline treatment for patients with advanced stage follicular lymphoma (FL), and it has been increasingly used over the last few years. However, limited data exist regarding the management and outcome of patients who relapse and/or progress after frontline R2.
Methods. In a retrospective study of patients with advanced stage FL grade 1-3A treated with frontline R2 at MD Anderson Cancer Center between 08/2008 and 01/2020, we examined treatment strategies and outcomes for patients who progressed/relapsed and required salvage therapy. Response was retrospectively assessed according to 2014 Lugano response criteria. Survival outcomes were calculated from time of first salvage therapy.
Results. Among 156 patients with advanced stage FL treated with frontline R2, 33 (21%) relapsed/progressed and required salvage therapy, with a median time to first salvage therapy of 33 months (range, 1-122 months); 12 (8%) patients relapsed within 24 months, and no patients showed transformation at time of first relapse. At time of first relapse/progression, 12 (36%) patients were older than 60 years, 19 (57%) were male, 9 (27%) had high risk FLIPI, and 1 relapsed with localized disease.
Overall, the median number of salvage systemic therapies after R2 was 1 (range, 0-4), and maintenance following any line of salvage was used in 7 (21%) patients; 1 (3%) patient each had autologous or allogeneic stem cell transplant, both had progressed within 24 months of frontline R2. First salvage therapy was: bendamustine with an anti-CD20 monoclonal antibody (MAb) in 8 (24%) patients, RCHOP in 8 (24%), an anti-CD20 MAb alone in 8 (24%), a clinical trial in 6 (18%), repeated R2 in 2 (7%), and radiotherapy (XRT) in 1 patient with localized disease. Thirty-two patients were evaluable for response after first salvage therapy: the overall response rate was 78% and complete response rate was 72%.
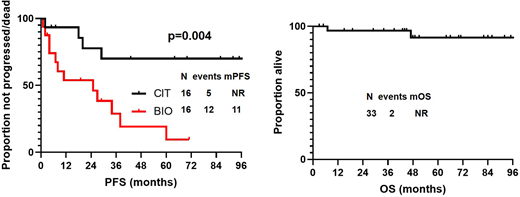
After a median follow-up of 51 months (95%CI, 27-75 months) from time of first salvage therapy initiation, 17 patients progressed/died, and median PFS was 38 months (95% CI, 1-82 months). None of the baseline characteristics collected at time of first salvage therapy were significantly associated with PFS. Median PFS was significantly longer in patients who received chemoimmunotherapy (CIT) as compared to biological therapy (BIO) at relapse (99 vs 25 months, p=0.004)(Figure). Transformation was identified in 2 (7%) patients, 2 and 20 months after initiation of first salvage therapy. At most recent follow-up, 2 (7%) patients died (1 of unknown cause, 1 of transformed FL), and median OS has not been reached (Figure). Second cancers (excluding transformation) were diagnosed in 1 (2%) patient after first salvage chemotherapy, and was represented by a pancreatic adenocarcinoma, after 74 months.
Discussion. Chemoimmunotherapy is an effective treatment strategy for patients with FL who relapse after frontline R2. The optimal salvage therapy for these patients needs to be prospectively investigated. Tissue samples derived from patients included in this study are being analyzed at our institution, and changes occurring in the tumor microenvironment are being characterized.
Westin:Kite: Consultancy; Genentech: Consultancy; 47 Inc: Consultancy; MorphoSys: Consultancy; Unum: Consultancy; Juno: Consultancy; Curis: Consultancy; Novartis: Consultancy; Janssen: Consultancy. Lee:Guidepoint Blogal: Consultancy; Celgene: Research Funding; Oncternal Therapeutics: Research Funding; Takeda: Research Funding; Seattle Genetics: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Aptitude Health: Speakers Bureau. Neelapu:Celgene: Other: personal fees, Research Funding; Bristol-Myers Squibb: Other: personal fees, Research Funding; Merck: Other: personal fees, Research Funding; Kite, a Gilead Company: Other: personal fees, Research Funding; Takeda Pharmaceuticals: Patents & Royalties; Cellectis: Research Funding; Adicet Bio: Other; Calibr: Other; Unum Therapeutics: Other, Research Funding; Poseida: Research Funding; Incyte: Other: personal fees; Precision Biosciences: Other: personal fees, Research Funding; Legend Biotech: Other; Acerta: Research Funding; Allogene Therapeutics: Other: personal fees, Research Funding; Karus Therapeutics: Research Funding; Pfizer: Other: personal fees; N/A: Other; Novartis: Other: personal fees; Cell Medica/Kuur: Other: personal fees. Vega:NCI: Research Funding. Fowler:TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding. Nastoupil:Karus Therapeutics: Research Funding; Gamida Cell: Honoraria; Celgene: Honoraria, Research Funding; Gilead/KITE: Honoraria; Novartis: Honoraria, Research Funding; Merck: Research Funding; TG Therapeutics: Honoraria, Research Funding; LAM Therapeutics: Research Funding; Pfizer: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Genentech, Inc.: Honoraria, Research Funding; Bayer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal